Delve Into Medical Micro Molding for Precision Components in Healthcare Devices
Delve Into Medical Micro Molding for Precision Components in Healthcare Devices
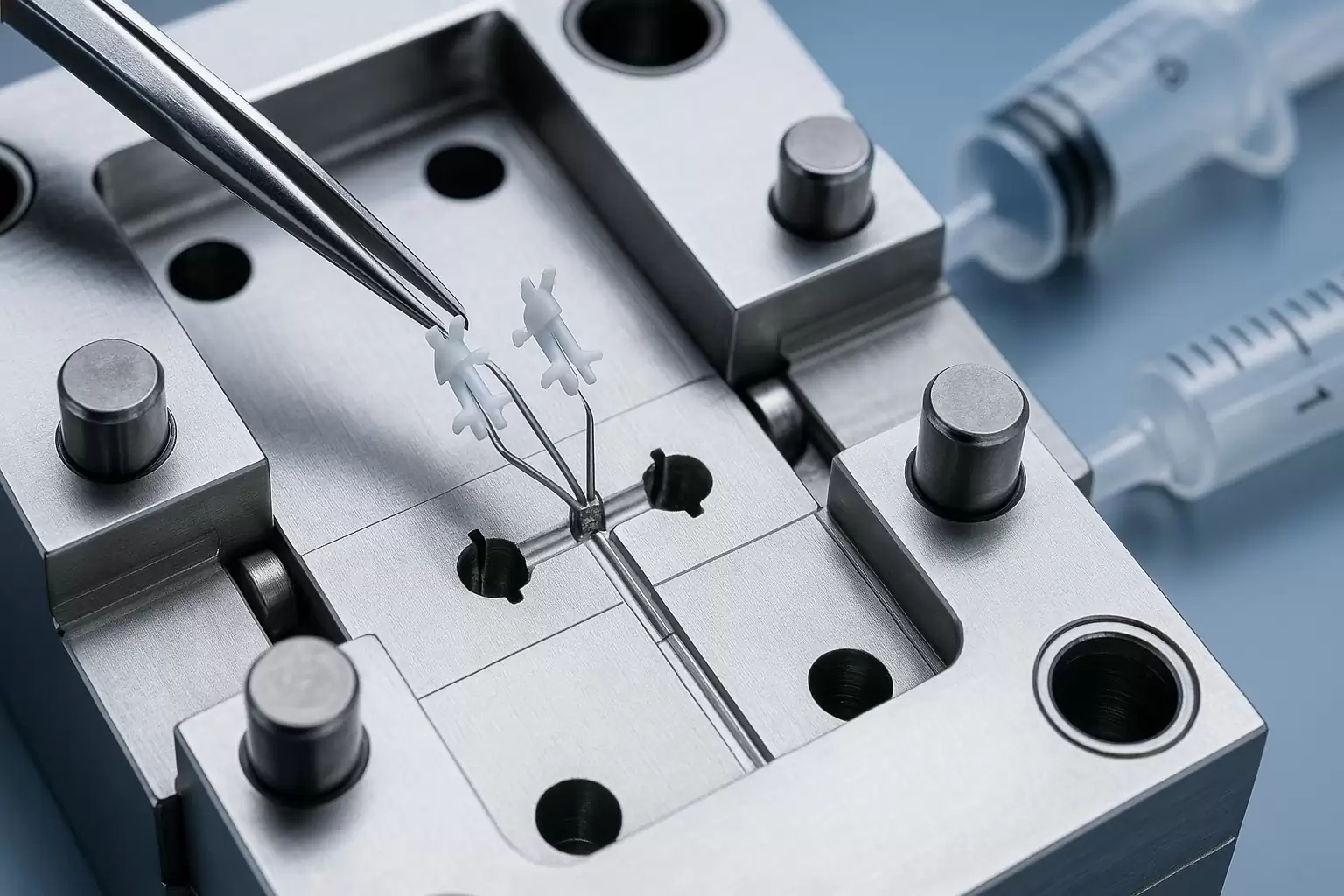
Catheter tips thinner than a grain of rice, PEEK gears that drive implantable pumps, and bio-resorbable anchors smaller than a sesame seed—all emerge from the exacting discipline of medical micro molding. By shrinking conventional injection-molding science to a sub-gram scale and pairing it with clean-room controls and med-grade polymers, manufacturers can deliver parts that meet ISO-13485 traceability and survive the rigors of sterilization, drug exposure, and human implantation. Below is an in-depth guide for R&D, quality, and sourcing teams exploring micro molding for next-generation healthcare devices.
1 | Why Micro Molding, Not Just “Small” Molding?
| Criterion | Conventional Small Part | True Micro Molded Part |
|---|---|---|
| Shot Size | 0.5 – 3 g | 0.05 – 0.3 g (or < 0.1 cm³) |
| Feature Size | ≥ 150 µm ribs | 10–50 µm orifices, 50 µm walls |
| Gate Diameter | 0.8 – 1.2 mm | 0.1 – 0.3 mm (sub-gate, hot tip) |
| Tolerance | ± 25–50 µm | ± 5–10 µm (single cavity) |
Micro molding presses run ultra-short, high-speed screws, deliver melt shots under 0.1 g, and measure injection velocity in mm/ms—not cm/s—ensuring polymer fills micro-channels before it freezes.
2 | Clean-Room Integration—Beyond White Walls
| Control Layer | Medical Requirement | Practical Implementation |
|---|---|---|
| Environment | ISO 14644-1 Class 7 or 8 for molding; Class 5–6 for assembly | HEPA-filtered laminar flow boxes over press clamp and conveyor |
| Material Handling | USP <661>, ISO 10993 certified resin lots | Closed drying hoppers with desiccant air to −40 °C dew point |
| Lot Traceability | 21 CFR Part 820 / ISO 13485 | Barcode-linked resin, tool cavity, machine parameters, and operator ID |
| Sterilization Compatibility | EtO, gamma, e-beam, or steam | Polymer supplier provides data; molder verifies no cosmetic shift after dose |
3 | Tooling Technology—Where Precision Begins
-
µ-EDM & µ-Milling: Electrodes < 70 µm cut steel details invisible to the naked eye.
-
Mirror-Polish Inserts: Ra < 0.02 µm for crystal-clear COC/COC flow channels.
-
Dedicated Hot Halves: Valve gates 0.15–0.25 mm eliminate vestige that could block micro-fluid paths.
-
Conformal Cooling: 3-D-printed cores stabilize cavity temp within ± 0.5 °C, critical at this scale.
Tip – An experienced micro-tool shop will hold core/cavity concentricity within 3 µm and validate with white-light interferometry.
4 | Material Selection for Micro Medical Parts
| Polymer | Key Traits | Typical Micro Component |
|---|---|---|
| PEEK (Med-grade) | 250 °C HDT, wear-proof | Catheter gear sets, implantable housings |
| LCP | Near-zero shrink, high modulus | 0.2 mm torque-limiter gears, sensor frames |
| COC / COP | Glass-like clarity, low extractables | Micro-fluidic chips, diagnostic cuvettes |
| PP (Random, USP VI) | Excellent flex-fatigue, EtO-stable | Living-hinge reagent caps, vials |
| Bio-Resorbable PLA/PLG | Degrades in vivo, drug-eluting friendly | Micro-anchors, tissue scaffolds |
5 | Process Window & Scientific Micro Molding
-
Injection Velocity: 200–500 mm/s (measured at a 0.2 mm gate)
-
Switch-Over Control: In-cavity pressure sensor at 95–98 % fill; critical for 5 µm wall repeatability.
-
Mold Temperature: 90 – 160 °C for semi-crystalline PEEK/LCP; narrow band prevents flash.
-
Screw Recovery: Complete within cooling time, else shear heats degrade resin IV at micro scale.
CpK targets ≥ 1.67 on dimensions < 100 µm require real-time cavity-pressure feedback and closed-loop servo control.
6 | Validation Pathway—IQ → OQ → PQ at the Micron Level
-
IQ: Verify µ-EDM electrode wear logs, mold steel hardness, and cavity finish.
-
OQ: DOE across melt temp, V/P, and mold temp; analyze replicate parts with CT-scan metrology.
-
PQ: Three 300-piece lots at nominal settings; full GD&T, leak, and bioburden tests.
CT-scanning is indispensable for non-destructive dimensional checks on micro features unseen by touch probe.
7 | Case Study—Implantable Pump Gear (Ø 3.2 mm, 20-Tooth PEEK)
| Metric | Traditional Molder | Micro Molder |
|---|---|---|
| Tooth Height Tolerance | ± 25 µm | ± 8 µm |
| CpK (Pitch Diameter) | 1.03 | 2.05 |
| Scrap Rate | 3.8 % | 0.4 % |
| Tool Change to Next Iteration | 4 weeks | 9 days |
| Sterilization Stability (gamma 25 kGy) | Moderate discoloration | No change ΔE < 0.5 |
Result: Device OEM met ISO 13485 submission timeline and reduced pump noise > 30 %.
8 | Why Choose TaiwanMoldMaker.com for Micro Molding
-
ISO 7 & 8 Clean-Rooms with automated take-out and enclosed conveyors.
-
Micro-Metrology Suite – CT-scan, laser confocal, and white-light interferometer.
-
100+ Med-Grade Polymers in Stock – PEEK, LCP, COC, PP-VI.
-
Prototype-to-Scale Credits – Aluminum micro tools credited toward H13 multi-cavity.
-
24/6 English-Fluent Engineering – Real-time DFM calls and eDart® data sharing.
???? Related Services
Ready to Go Micro?
Upload your CAD and CTQ list to TaiwanMoldMaker.com for a 48-hour feasibility report, DFM pack, and timeline—plus an offer on prototype micro tooling to kick-start your high-precision healthcare innovation.